what would happen to a cell of a colonial organism if you separated it from the other cells?
Introduction
The evolution from a fertilized egg jail cell, the and then-called zygote, to an embryo made up past hundreds of cells or to a juvenile and adult consisting of more than thousands to billions of cells is a hallmark of animals (Metazoa). Metazoan evolution is a complex process that is facilitated by the highly coordinated interplay of several not less complex sub-processes such as prison cell sectionalization (cleavage), cell–cell interaction, prison cell migration, and jail cell differentiation (Fairclough et al., 2013; Alberts et al., 2014; Brunet and King, 2017). The result of this interplay is a multicellular organism consisting of functionally specialized cells, so-chosen jail cell types. Diverse cell types are described in non-bilaterian metazoans such equally sponges (Porifera), comb jellies (Ctenophora), Trichoplax (Placozoa), and jellyfish (Cnidaria) (Sebé-Pedrós et al., 2018a, b). If these jail cell types appear in an ontogenetic sequence they are called temporal cell types. Temporal jail cell types are non restricted to Metazoa, but tin besides be plant in unicellular organisms where cells transition betwixt different prison cell types during life history (Mikhailov et al., 2009). However, in animals many different cell types are present during the same ontogenetic period. These cell types are then chosen spatial cell types. In bilaterian metazoans many spatial cell types are highly specialized and sometimes exert only one specific function (Wagner, 2014). In non-bilaterian metazoans spatial cell types are oftentimes multifunctional such as epithelial musculus cells in cnidarians, pinacocytes in sponges (both protection, contraction), and the "ocellus" in sponge larvae, a unmarried cell that performs locomotor (steering), photoreceptive, and pigmentation functions (Wagner, 2014).
Another multifunctional jail cell type is the collar prison cell, a polarized jail cell with an upmost flagellum surrounded by a microvillar collar (Leadbeater, 2015; Arendt et al., 2016; Brunet and Rex, 2017; Arendt et al., 2019). Collar cells are present in well-nigh all metazoans and their closest relatives, the choanoflagellates (Brunet and Male monarch, 2017; Laundon et al., 2019; Figure 1A). The colony-forming choanoflagellate Salpingoeca rosetta (Dayel et al., 2011) has emerged as a promising model organism to investigate the evolutionary origin of metazoan multicellularity and cell differentiation (Hoffmeyer and Burkhardt, 2016). Not only is S. rosetta easy to culture in the laboratory with a short generation time of 6–8 h and colony consecration is highly reproducible, information technology also has a fully sequenced transcriptome and genome and a suite of functional techniques are now available (Levin et al., 2014; Berth et al., 2018; Wetzel et al., 2018). S. rosetta exhibits a complex life history including different temporal cell types during unicellular and colonial life history changes (Dayel et al., 2011; Laundon et al., 2019; Figure 1B). Similar to metazoans, colonies of Due south. rosetta grade by mitotic divisions from a single founder cell. Cells within a rosette colony are held together by intercellular cytoplasmatic bridges, filopodia, and an extracellular matrix (Laundon et al., 2019). Rosette colony formation is induced by rosette inducing factor (RIF), a sulfonolipid secreted by the bacterium Algoriphagus machipongonensis (Alegado et al., 2012; Woznica et al., 2016).
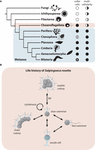
Effigy one. (A) Phylogenetic tree showing Choanoflagellata every bit sis group of the Metazoa (Steenkamp et al., 2005; Carr et al., 2008; Ruiz-Trillo et al., 2008; Adl et al., 2019). In addition, the presence (black circumvolve) and absence (white circle) of neckband cells and multicellularity beyond lineages are shown (Brunet and King, 2017; Laundon et al., 2019). The white asterisk indicates independent secondary losses. Half-filled circles indicate multicellularity in simply some species. In Filasterea, multicellularity is achieved by assemblage of single cells (half-filled white-gray circle) instead of clonal division. (B) Life history of the choanoflagellate South. rosetta afterward Dayel et al. (2011). RIF, rosette inducing cistron (Alegado et al., 2012).
Whether cells of a rosette colony correspond a cluster in which cells are identical to each other or differ from each other is still unclear. Although bulk transcriptomic analyses accept shown nearly identical expression patterns for unmarried and colonial cells in S. rosetta (Fairclough et al., 2013), a recent study described a singled-out morphology of cells in some South. rosetta colonies indicating differences of cells within choanoflagellate colonies (Laundon et al., 2019). Understanding whether private cells of a choanoflagellate colony are identical to each other or different from each other is important for a better understanding of the evolutionary origin of spatial cell differentiation and spatial cell types in the Metazoa. At that place are several theories on the evolutionary origin of metazoan multicellular development, cell differentiation, and cell types (Fairclough et al., 2010; Sebe-Pedros et al., 2017). Every bit proposed by Haeckel (1874) under the term "Blastaea/Gastraea theory," animals evolved through "…repeated self-division of [a] primary cell,…" (Haeckel, 1892). In this scenario, the last common ancestor of the Metazoa originated from incomplete cell sectionalization of a primary unmarried cell that formed a ball-shaped colony called a "Blastaea" consisting of identical cells (Haeckel, 1874). During evolution, intra-colonial division of labor led to increasing differences between cells resulting in specialized cells representing distinct cell types (King, 2004). In this Blastea hypothesis, spatial prison cell differentiation evolved before temporal cell differentiation in the stalk lineage of the Metazoa (Mikhailov et al., 2009). A hypothesis that contradicts Haeckel'south Blastea hypothesis was proposed by Zakhvatkin (1949). The so-chosen "Synzoospore theory" claimed that metazoans evolved from a unicellular ancestor that showed a multifariousness of different cell types during different life history stages. According to this theory, temporal cell differentiation was already present and accompanied by spatial cell differentiation in the metazoan stem lineage (Mikhailov et al., 2009).
In this study, we used ultrathin transmission electron microscopy (ssTEM) series sections of whole rosette colonies of S. rosetta to prepare three-dimensional (3D) reconstructions and measure volumes of prison cell bodies, nuclei, nutrient vacuoles, and mitochondria of 40 individual colonial cells from four colonies. Nosotros chose these structures because they tin exist precisely extracted digitally from the rest of the cellular components at the available resolution (in contrast to other cellular components such as the endoplasmic reticulum, vesicles, the Golgi apparatus, and glycogen granules, etc.). The nuclear and mitochondrial volumes are correlated with jail cell volume in a diversity of unicellular eukaryotes and metazoan prison cell types (Chan and Marshall, 2010). Nevertheless, until at present such a correlation has not been tested in choanoflagellates. In this context, a deviation of the nuclear volume ratio in some cells could bespeak a difference in transcriptional activity in cells within a colony (Chan and Marshall, 2010; Naumova and Dekker, 2010; Jevtić et al., 2014). Food vacuoles on the other manus seem to be more dynamic correlated with food supply rather than cell volume. We therefore expected a lower correlation of this organelle with prison cell book. Nosotros compared the results with available information (Laundon et al., 2019) on the cellular beefcake of choanocytes of the homoscleromorph sponge Oscarella carmela (Ereskovsky et al., 2017). The comparison of cells within a colony and between colonial choanoflagellate cells and sponge choanocytes will help to reveal whether (ane) cells in Southward. rosetta rosette colonies are indeed identical (presence of spatial jail cell disparity), (2) how variable the volume ratios of different cellular organelles are within complete rosette colonies (degree of spatial disparity), and (iii) if a like degree of spatial prison cell disparity is present in sponge choanocytes (representing a spatially distinct cell type).
Materials and Methods
3D Reconstructions of Complete Due south. rosetta Colonies
A summary of the workflow is shown in Supplementary Figure S1. For our analysis, we used digital image stacks of TEM sections of complete S. rosetta colonies (RC1–RC4; n = forty cells), previously published by Laundon et al. (2019) and bachelor from figshareone. The paradigm stacks were imported into AMIRA (FEI Visualization Sciences Grouping) and aligned manually. After, the jail cell body and major cell organelles (nucleus, mitochondria, and food vacuoles) were segmented manually. For surface reconstructions, surface models were rendered from the segmented materials, numbers of polygons were reduced and the surfaces were smoothened for the first time. Materials were so imported into Maya (Autodesk), smoothened twice, and colored for last prototype rendering. For book renderings, segmented materials were subtracted from the main prototype stack and exported as separate image stacks. Book renderings of cells and organelles were prepared using VG Studio Max 2.0 (Volume Graphics).
Surface Measurements and Book Calculations
Separated image stacks of cell bodies, nuclei, mitochondria, and nutrient vacuoles of the cells of RC1–RC4 were analyzed with Fiji. Image stacks were imported and masked to create a binary epitome of the jail cell body or organelle (black) confronting a white background. The number of black pixels was counted on each section. The scale bar imprinted in the images was measured in Fiji cartoon a line of analogous length. The length of this line in pixels was then divided by the concrete length of the scale bar to calculate the physical size for each pixel. All surface surface area analyses were conducted using unsmoothed, unprocessed materials. Later, surface area measurements were exported to Microsoft Excel 2010 (Microsoft Corporation) and volumes were calculated by multiplying each surface value with the section thickness (RC1: 70 nm; RC2–RC4: 150 nm) and book ratio calculations and diagrams were prepared.
Results
Nuclear Book Correlates With Cell Size in Due south. rosetta Rosette Colonies
In most cells of the 4 analyzed rosette colonies, the nucleus is located approximately in the middle of the apical–basal axis of the cell (Figure 2A and Supplementary Figures S6–S9). For the relative and absolute volume calculations all sub-structures of the nucleus (the nuclear lamina, eu- and heterochromatin, and the nucleolus) were included (Table ane). A plot of the accented nuclear volume against the prison cell book is shown for each colony in Figure ii.
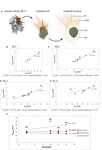
Effigy 2. (A) 3D-volume-renderings to illustrate the position and size of the nucleus in a colonial S. rosetta jail cell. (B–E) Plots of absolute nuclear volumes against the accented cellular volume of cells from the four rosette colonies investigated in this study (RC1–RC4). (F) Plot of the minimum (crimson), mean (black) and maximum (bluish) relative nuclear volume of each of the 4 rosette colonies. Cells are color coded according to Table 1. Vnumax, maximal nuclear book; Vnumin, minimal nuclear volume.

Table 1. Accented and relative nuclear volumes of cells of RC1–RC4.
The relative mean nuclear volumes range from xiv.77 (RC1) over 14.95% (RC2) and 15.9 (RC3) to xvi.32% (RC4). The maximum volume differences between cells inside a colony range from two.78 (RC1) over iv.43% (RC2) and xiii.2 (RC3) to 17.17% (RC4). The high maximum volume differences in RC3 and RC4 are mainly due to the large nuclear volume ratios in the carrot-shaped (RC3) and chili-shaped cell (RC4).
In summary, a relatively strong correlation betwixt the nuclear volume and the total cell volume can be recognized (Figures 2B–Eastward). In cells of RC1 (Figure 2B) and RC2 (Figure 2C), the correlation betwixt nuclear volume and total prison cell volume is strongest. In RC4, the correlation between nuclear and total cell volume is the everyman among the four colonies analyzed. This is again mainly due to the exceptionally high relative nuclear volume in the chili-shaped cell (Figure 2E; black asterisk). The plot of the relative hateful, minimal, and maximal nuclear volumes against colony size indicates a higher cell disparity in larger colonies (RC2, RC3, and RC4) compared to RC1 (maximum difference; Figure 2). Still, intracolonial cell disparity seems not to increment in a stepwise mode. The minimal and hateful relative nuclear volumes do non show a high variation between the colonies, most of the variation comes from the maximal relative nuclear volumes.
Mitochondrial Book Correlates With Prison cell Size in South. rosetta Rosette Colonies
Most mitochondria in single-cell and colonial Southward. rosetta are organized within a network, called the mitochondrial reticulum, surrounding the nucleus (Leadbeater, 2015; Figure 3A and Supplementary Figures S6–S9). Just the relative and absolute volume of mitochondria located in the cytoplasm of each prison cell of RC1–RC4 were regarded as functional and reconstructed (Table 2). A plot of the absolute mitochondrial book against the cell volume is shown for each colony in Figure 3. Mitochondria incorporated into nutrient vacuoles were regarded as non-functional and were not reconstructed.
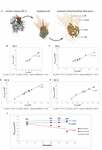
Figure 3. (A) 3D-volume-renderings to illustrate the mitochondrial reticulum in a colonial Southward. rosetta prison cell. (B–Eastward) Plots of accented mitochondrial volumes against the absolute cellular volume of cells from the four rosette colonies investigated in this report (RC1–RC4). (F) Plot of the minimum (red), mean (black) and maximum (bluish) relative mitochondrial book of each of the four rosette colonies. Cells are color coded according to Table two. Vmtmax, maximal mitochondrial volume; Vmtmin, minimal mitochondrial book.

Table two. Absolute and relative mitochondrial volumes of cells of RC1–RC4.
The relative mean mitochondrial volumes range from 5.81 (RC1) over half-dozen.02% (RC4) and 6.05 (RC2) to 6.24% (RC3). The maximum volume differences between cells inside a colony range from 1.09 (RC1) over 1.67% (RC2) and ii.2 (RC3) to 3.66% (RC4). The higher maximum book differences in RC3 and RC4 are again mainly due to the low mitochondrial book ratios in the carrot-shaped (RC3) and chili-shaped cells (RC4).
In summary, our data indicate a stiff correlation between the mitochondrial volume and the total prison cell book in cells of each colony (Figures 3B–East). The relative mean mitochondrial volume increases only slightly with colony size. The relative maximal mitochondrial book is lowest in RC1 while virtually like in RC2, RC3, and RC4. We observed that in all colonies the majority of the mitochondria of a jail cell are organized equally one large mitochondrial reticulum and simply a few solitary mitochondria can be observed. However, an exact measurement of the number of mitochondria was not possible due to the department thickness of 150 nm (in RC2, RC3, and RC4). This thickness in combination with slight distortion artifacts from the sectioning process did non always allow reliable decisions equally to whether one mitochondrium is continuous from i section to another or if it ends and another one begins in the following section.
Food Vacuole Volume Does Not Correlate With Cell Size in Southward. rosetta Rosette Colonies
In most cells food vacuoles are located in the basal half along the apical–basal axis of the jail cell (Figure 4A and Supplementary Figures S6–S9). In the TEM sections analyzed, nutrient vacuoles appear in different electron densities from high (dark gray) to relatively low (light gray). In between the two "extremes," food vacuoles appear in different electron densities represented by dissimilar shades of gray. The electron density might correspond different stages in the digestive wheel. To clarify the complete volume of food vacuoles within a cell we included all recognizable food vacuoles irrespective of their electron density (Table iii). A plot of the absolute food vacuole book against the prison cell volume is shown for each colony in Figure 4.
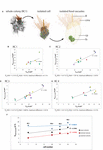
Figure 4. (A) 3D-volume-renderings to illustrate the position and size of some food vacuoles in a colonial S. rosetta cell. (B–E) Plots of absolute food vacuole volumes against the absolute cellular volume of cells from the four rosette colonies investigated in this study (RC1–RC4). (F) Plot of the minimum (reddish), hateful (black) and maximum (bluish) relative food vacuole book of each of the four rosette colonies. Cells are color coded co-ordinate to Table 3. Vfvmax, maximal food vacuole book; Vfvmin, minimal food vacuole volume.

Table iii. Absolute and relative food vacuole volumes of cells of RC1–RC4.
The relative mean food vacuole volumes range from iv.81 (RC1) over 4.93% (RC4) and 6.29 (RC2) to seven.32% (RC3). The maximum volume differences between cells inside a colony range from three.13 (RC4) over 3.3% (RC2) and v.35 (RC3) to six.xi% (RC1).
In summary, our data indicate that at that place is simply a weak correlation between nutrient vacuole volume and the total cell volume in cells of each colony (Figures 4B–E). A plot of the relative mean, minimal, and maximal food vacuole volumes confronting the colony size indicates that the maximum book divergence of food vacuoles is independent from colony size (maximum difference; Figure four). An exact measurement of the number of food vacuoles was not possible due to the same limitations mentioned for the measurement of the mitochondrial number.
Cells Within Rosette Colonies of Due south. rosetta Showroom a Variety of Different Morphologies
The private cells of the four investigated rosette colonies (RC1–RC4) exhibit a diverseness of volumes/sizes and morphologies (Figure 5). 3D reconstructions of all cells of RC1–RC4 are depicted in Supplementary Figures S2–S5. Many cells exhibit an ovoid morphology, slightly elongated along the apical–basal axis (AB-axis) (Figure 5A). However, some cells showroom a more roundish (Figure 5B) or ovoid shape horizontally to the AB-axis (Figure 5C). Laundon et al. (2019) described ii cells with a distinct morphology within rosette colonies, C5 of RC3 (carrot-like jail cell; Figure 5D) and C5 of RC4 (chili-like prison cell; Effigy 5E). All cells within a rosette colony exhibit a variety of cell membrane protrusions such equally filopodia, pseudopodia, and larger lobopodia-similar protrusions that might correspond pine- and/or endocytotic events (Figures 5F–I).
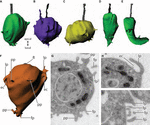
Figure 5. (A–F) 3D-surface-reconstructions of cells from four different S. rosetta colonies. Cell sizes are not to scale. (A) Ovoid morphology (RC1, C6). (B) Roundish morphology (RC3, C10). (C) Ovoid morphology with the ovoid centrality horizontally to the apical–basal axis (RC1, C3). (D) "Carrot"-cell (RC3, C5). (E) "Chili"-cell (RC4, C5). (F) Different types of membrane protrusions within rosette colonies (RC1, C2). (G–I) TEM sections of different types of membrane protrusions. ec, endo- or pinocytosis; fl, flagellum; fp, filopodium; lp, lobopodium; pp, pseudopodium.
In RC1 (seven cells; Supplementary Figure S2), cell volumes range from 15.98 (C2) to 37.71 μmthree (C5). Five cells exhibit a more than ovoid morphology. Three of these cells (C1, C2, and C6) are slightly elongated forth the AB-axis. The other two cells (C3 and C4) are elongated horizontally to the AB-axis. Ii cells (C5 and C7) evidence a more roundish shape.
In RC2 (11 cells; Supplementary Figure S3), jail cell volumes range from 19.12 (C11) to 46.58 μm3 (C10). Ix cells exhibit a more than ovoid morphology. Eight of these cells (C1, C2, C4, C5, C6, C7, C9, and C10) are slightly elongated along the AB-axis. C8 is elongated horizontally to the AB-axis. Two cells (C3 and C11) evidence a more than roundish shape.
In RC3 (12 cells; Supplementary Effigy S4), cell volumes range from 10.2 (C5) to 51.24 μm3 (C7). 9 cells exhibit a more ovoid morphology. 8 of these cells (C1, C3, C5, C6, C7, C8, C9, C11, and C12) are slightly elongated along the AB-axis. Equally previously reported, C5 exhibits a distinct slender, carrot-shaped morphology (Laundon et al., 2019). C4 is elongated horizontally to the AB-centrality. Ii cells (C2 and C10) are more roundish. C7, the largest prison cell of the colony, and C12 shows an exceptional loftier number of membrane protrusions (Supplementary Figure S4).
In RC4 (10 cells; Supplementary Figure S5), cell volumes range from 13.98 (C5) to 36.47 μmthree (C8). 7 cells (C1, C3, C4, C6, C7, C9, and C10) showroom a more than ovoid morphology, slightly elongated along the AB-axis. As previously reported, C5 exhibits a distinct slender, chili-shaped morphology (Laundon et al., 2019). 2 cells (C2 and C8) prove a more roundish shape.
To determine if specific jail cell morphologies correspond to specific nuclear, mitochondrial, and food vacuoles volumes we plotted cell morphologies against the full cellular book and volumes of the investigated organelles (Figure half-dozen). No profound differences of full prison cell book, nuclear, mitochondrial, and food vacuole book were plant except for the carrot-shaped and chili-shaped cells (Figures 6A–D). Subsequently, we plotted the relative full cellular, nuclear, mitochondrial, and food vacuole volumes against each other to examination if there are specific patterns for the distinct cell morphologies (Figures 6E–J). There is a strong correlation of the nuclear and mitochondrial volumes with the total jail cell volume. This confirms our earlier observations where the 4 colonies were considered separately. The horizontally ovoid cells notwithstanding exhibit a lower correlation of nuclear volume to cell volume compared to the other cell morphologies. Regarding the nutrient vacuole to jail cell book ratio, the overall correlation was much lower compared to nuclear and mitochondrial volumes. The lowest correlation of food vacuole to prison cell book ratio can be establish in roundish cells (Effigy 6G). No correlations were establish when nuclear, mitochondrial, and food vacuole ratios were plotted against each other (Figures 6H–J).

Figure 6. (A–D) Plots of the different types of prison cell morphology of colonial South. rosetta cells against cellular volume and relative organelle volumes. (E–J) Plots of the accented (E–M) and relative organelle volumes (H–J) against each other. A regression line is shown in the same color as it corresponding type of jail cell morphology. The black regression line in panels E–G is calculated from all 40 cells.
Quantitative Analysis of Prison cell–Cell Contacts Reveals Plasma Membrane Contacts in Colonial Cells of S. rosetta
Numerous intercellular bridges and filo-/pseudopodial contacts can be found between cells in a colony (Figure 7; Leadbeater, 2015; Laundon et al., 2019). Additionally to our best knowledge, we report plasma membrane contacts between some cells of a colony for the first time (Figures 7A,B). These membrane contacts are found in all iv colonies and range from relatively minor areas (effectually 100 nm length on a section) up to areas of a length of >500 nm (length on a section).
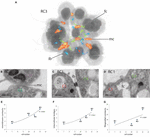
Figure 7. (A) 3D-volume-rendering of South. rosetta rosette colony RC3 to illustrate the distribution of cell–cell contacts within a colony. (B–D) TEM sections of unlike colonies highlight various types of cell–cell contacts in colonial S. rosetta cells. (B) Plasma membrane contact betwixt C5 and C10 (RC3). (C) Intercellular bridge between C1 and C4 (RC1). (D) Filopodial contact betwixt C1 and C4 (RC1). (Eastward–G) Plots of the number of specific jail cell–prison cell contacts against the colony size (measured in cell number). ib, intercellular bridge; fc, filopodial contact; mc, membrane contact.
We quantified the number of the newly plant plasma membrane contacts in the colonies used in this written report. It seems that the number of plasma membrane contacts increases with the colony size (Figure 7E). This is similar to the number of intercellular bridges (Figure 6F; Laundon et al., 2019). The number of filopodial/pseudopodial contacts between cells within the colonies seems not correlated with colony size (Figure 7G). A detailed summary of the types (intercellular bridges, membrane contact, and filopodial contact) and number of connections of individual cell of each of the four investigated colonies is given in Table 4.

Table four. Types of cell–jail cell contacts of cells of RC1-RC4.
Discussion
In this report, we analyzed cell morphologies, volumes of cell bodies, and volumes of some major organelles (nucleus, mitochondria, and nutrient vacuoles) of four S. rosetta rosette colonies (40 cells in total). The aims were: (1) To investigate whether cells in rosette colonies of S. rosetta are indeed identical or if they differ from each other. (two) In case they differ from each other, to what degree do they vary in terms of morphology, cell volume, and organelle content? (3) To compare the intracolonial cell differences to the differences within a group of choanocytes of the homoscleromorph sponge O. carmela. The differences of cells inside a colony are here described in a relative fashion using the term "prison cell disparity" (indicated by maximum volume differences in this study). Identical cells show no disparity at all, the maximum volume difference within a colony/tissue is zero. In contrast, cells that exhibit maximum volume differences atomic number 82 to a sure caste of cell disparity within a colony/tissue (Figure viii).
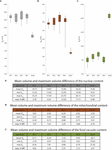
Figure 8. (A–C) Box and whisker plots of the relative volumes of different prison cell organelles of S. rosetta colonies (RC1–RC4) and 5 choanocytes of O. carmela (Ocar). Values in the tables are given in % in relation to the total cellular book. Asterisk, data taken from Laundon et al. (2019). (A) Nuclear volumes (gray box). (B) Mitochondrial volumes (brown box). (C) Food vacuole volumes (green box). (A'–C') Tables showing the minimum, maximum, and mean volumes also as the maximum volume differences for each of the investigated organelles. max. V diff., maximum volume deviation; hateful 5, mean volume; Vmax, maximal volume; Vmin, minimal volume.
Rosette Colonies of S. rosetta Exhibit Spatial Cell Disparity Regarding Cell Morphology and the Nuclear and Mitochondrial Content
Cells within South. rosetta colonies are non identical just prove spatial cell disparity regarding their nuclear, mitochondrial, and food vacuole contents (Figure 8). The largest deviation of the nuclear and mitochondrial contents is found in the carrot-shaped (RC3) and chili-shaped cells (RC4). If these two cells are removed from the assay the overall cell disparity declines in these two colonies (RC3: Fivenu from 13.2 to 4.43%, Fivemt from 2.two to ane.one%; RC4: Vnu from 17.17 to ii.59%, Fivemt from three.66 to 0.77%). In this regard the carrot-shaped and the chili-shaped cells are the main reason for the cell disparity observed in RC3 and RC4. However, since it is not known whether the carrot- and chili-shaped cells exert special functions within a colony it cannot be excluded that other cells within the colony would compensate for the removal of these cells by internal organelle changes. Due to the lack of noesis if in that location is a functional role of the carrot-shaped and chili-shaped cells nosotros consider all cells integrated within a colony and discuss our results with the carrot-shaped and chili-shaped cell included in the analysis.
The maximum book differences of the nuclei (Figure 8A) could indicate slightly different transcription activities of cells within a colony. It is known that changes in nuclear size and form can exist the cause or issue of changes in chromatin organization, gene expression, and other physiological processes of the cell (Naumova and Dekker, 2010; Jevtić et al., 2014). A study on myotube formation in human being myoblasts has shown that a decrease in nuclear size is correlated with contradistinct histone modifications, chromatin remodeling, and cistron silencing (Rozwadowska et al., 2013). The comparison of nuclear volumes of colonial cells (this study) with single cells of S. rosetta (Laundon et al., 2019) shows no difference between the two life history stages. Therefore, the observed differences of the relative nuclear volumes (eastward.g., in the carrot-shaped and chili-shaped cells or the horizontally ovoid cells) might be due to asynchronous cell-cycles, different metabolic states, or even different intra-colonial oxygen levels affecting transcriptional action (Zhou et al., 2007) rather than different jail cell types within cells of a colony. The large difference of the relative nuclear volumes of the carrot-shaped and chili-shaped cell could result from a loftier asynchrony in cell-bicycle/metabolism compared to the other cells in the colony or, in this special case, point more specialized function of these ii cells.
Maximum book differences, resulting in spatial cell disparity, were also observed for the relative mitochondrial (Figure 8B) and nutrient vacuole volumes (Effigy 8C). Laundon et al. (2019) suggested that there is a significant difference in mitochondrial number (single cells: 25.iii ± 5.eight vs. colonial cells: four.3 ± iv.2) but not mitochondrial book (unmarried cells: 5.08 ± 1.14% vs. colonial cells: 6.63 ± 0.42%) betwixt unmarried and colonial S. rosetta cells. This difference in number could exist due to a higher demand on free energy necessary for locomotion in single-prison cell South. rosetta. We confirm the results from Laundon et al. (2019) regarding the relative mean volume of the mitochondrial reticulum in colonial South. rosetta (RC1: 5.81%; RC2: 6.05%; RC3: 6.24%; RC4: 6.02%; Figure 8B). Our reconstructions of the mitochondrial reticulum of colonial cells also support the presence of a lower number of mitochondria in colonial cells (Laundon et al., 2019). All the same, it was non possible to make up one's mind the verbal number of mitochondria in cells of RC2, RC3, and RC4 due to the thickness (150 nm) of the sections. The lower mitochondrial number in colonial cells could exist due to mitochondrial fusion. It is known that mitochondrial fusion is stimulated by energy demand and stress while fission may generate new organelles and facilitates quality control (Youle and Van Der Bliek, 2012). Limited mitochondrial fusion results in improper embryogenesis and is associated with some human diseases (Chen and Chan, 2010). Therefore, mitochondrial fusion might act as a "defense mechanism" against cellular aging (Westermann, 2002). Similar to our speculation on the variety of cell morphologies, the absence of extensive directed locomotion and the decreasing need for loftier free energy consumption in colonies might release cells from the constraint of having a high number of agile, ATP-producing mitochondria. This may permit for a higher degree of mitochondrial fusion and increased longevity of mitochondrial function (Chen and Chan, 2010). The nutrient vacuoles are regarded every bit the about dynamic organelle type investigated in this study. The observed maximum volume differences of the food vacuole content of cells inside colonies range from 3.13 to 5.35%. This divergence might be about likely due to different metabolic rates and differences in the nutrient uptake of private cells.
Colonies show a multifariousness of cell morphologies from roundish over ovoid (along the AB-centrality or horizontal to the AB-axis) to ii extreme morphologies, the carrot-shaped (RC3; Laundon et al., 2019) and the chili-shaped prison cell (RC4; Laundon et al., 2019).
Nosotros translate our results that cells within a colony showroom spatial cell disparity most likely on the basis of asynchronous cell cycles and different metabolic rates. The carrot-shaped and chili-shaped cells with their larger nuclear and lower mitochondrial content might already exert specialized functions compared to other cells inside the same colony.
Rosette Colonies of S. rosetta Exhibit Slightly Higher Spatial Cell Disparity Compared to Sponge Choanocytes
A comparison of the nuclear volume data of colonial S. rosetta cells with data from choanocytes of the homoscleromorph sponge O. carmela (Laundon et al., 2019) shows that the mean relative nuclear volume of O. carmela choanocytes (ix.78%, due north = five; Figure 7A) is around ane-tertiary lower as the mean nuclear volume in cells of Due south. rosetta colonies (eastward.g., RC1: 14.77%, n = vii; Figure 8A). Additionally, the maximum volume departure of the nuclear book of O. carmela choanocytes (within the aforementioned choanocyte chamber of a sponge private) is almost 3 times lower as the smallest maximum volume difference within one of the analyzed S. rosetta colonies (due east.g., RC1: maximum book difference = 2.78%; Figure 8A). These differences in the relative nuclear book tin exist explained in ii ways. The first explanation focuses on alterations of the nuclear book due to cell division. For case, in the demosponge Hymeniacidon sinapium, choanocytes dissever every 20–xl h (Shore, 1971). It is interesting to notation hither that archaeocytes of another demosponge, Amphimedon queenslandica, differentiate into choanocytes inside only 2 h without prior cell division (Sogabe et al., 2019). Cells of Southward. rosetta rosette colonies in contrast split every 6–eight h (Fairclough et al., 2010). Due to the shorter cell bicycle length of choanoflagellates compared to sponges information technology might be that the fixed S. rosetta colonies independent, by chance, more cells in the G2-stage of the jail cell bike. G2-stage nuclei are oftentimes larger than nuclei during for instance the G1-stage due to the doubling of the Deoxyribonucleic acid during the S-stage preceding the G2-phase (Maeshima et al., 2011). The second caption focuses on the specialized part of sponge choanocytes. Colonial choanoflagellate cells have been regarded equally being more or less similar significant that they bear witness no real division of labor as present in Metazoa (Leadbeater, 2015). Therefore choanoflagellate cells have to be "all-rounder" and constitutively express a multifariousness of cellular modules such as for instance a ribosome biogenesis module, a flagellar module, a contractility module, and a filopodia/microvilli module (Brunet and Rex, 2017) to encounter all possible functional demands. Choanocytes in contrast, existing in a multicellular organism with other prison cell types, are specialized on just a few functions such as creating water current and food uptake (Simpson, 2012; Mah et al., 2014; Dunn et al., 2015). Therefore, sponge choanocytes may not express a multitude of different cellular modules. Instead they might express only some modules in a cell type specific mode (Brunet and Male monarch, 2017; Figure 9). The expression of fewer cellular modules could be reflected by a decreased number of active genes and higher values of densely packed heterochromatin resulting in a smaller relative nuclear volume. Specialization could also explicate the lower cell disparity in O. carmela choanocytes compared to colonial cells of S. rosetta. These arguments tin exist tested by investigating the chromatin architecture and euchromatin/heterochromatin ratios in "all-rounder" colonial cells of South. rosetta and specialized choanocytes of O. carmela.

Figure 9. Hypothesis on spatial and temporal cell disparity in Due south. rosetta single cells, colonies, and metazoans (e.grand., sponges). Three cell types are described in solitary life history stages of Southward. rosetta (Dayel et al., 2011). Each of the solitary prison cell types might exhibit distinct expression levels of several constitutive cellular modules (sensu Brunet and Male monarch, 2017) and cell disparity varies simply in fourth dimension simply not in space. In colonial South. rosetta, cell disparity additionally varies in space. Upon increase of colony size the probability in cell disparity increases. In metazoans, jail cell number increases tremendously leading to a high degree of cell disparity. During development (and life history), cells differentiate into singled-out jail cell types that express a specific set of cellular modules. This process decreases prison cell disparity between cells of the same jail cell types only increases prison cell disparity between cells belonging to different cell types.
The view of choanoflagellate cells as "all-rounders" and sponge choanocytes as functional specialists is farther supported by the two times higher hateful mitochondrial book and the almost 4 times lower mean food vacuole book of colonial Southward. rosetta cells compared to O. carmela choanocytes (Figure 8B). As mentioned by Laundon et al. (2019) choanocytes as specialized cells without locomotory function might not need such loftier amounts of energy as free swimming S. rosetta cells with dual functions (food acquisition and locomotion) do. Additionally, we find that the maximum book deviation of the mitochondrial volumes of cells within S. rosetta colonies is effectually one.5–4.8 times higher compared to the analyzed O. carmela choanocytes of the same individual (Figure 8B). The prison cell disparity in choanoflagellate colonies could indicate early stages of "division of labor" (Bonner, 2009). Cells of a colony are connected by intracellular bridges, pseudo-/filopodia (Dayel et al., 2011; Leadbeater, 2015; Laundon et al., 2019) and membrane contacts (this study). Some of these structures could serve in exchange of metabolic compounds and could explain that certain cells within a colony must reduce the cellular volume devoted to mitochondria and increase the expression of other "cellular modules" (sensu Brunet and Male monarch, 2017) while others increase the mitochondrial volume to encompass the total energetic demands of the colony. Of special interest would exist a comparison of the mitochondrial and food vacuole volumes between sessile, thecate S. rosetta and sponge choanocytes. If the "loss of constraints" hypothesis is right, sessile Southward. rosetta should exhibit higher volumes of nutrient vacuole and lower mitochondrial volumes than "boring and fast swimmer" cells and therefore exist more similar to choanocytes.
Plasma Membrane Contacts in South. rosetta Rosette Colonies
Jail cell–jail cell contacts and differential cell adhesion are key features during development and morphogenesis of whatsoever metazoan embryos (Gilbert, 2013). These contacts tin can exist established in different ways utilizing intercellular bridges, filo-/pseudopodia and/or whole areas of the cell membrane. Intercellular bridges have been described in S. rosetta and several colony-forming choanoflagellates (Karpov and Coupe, 1998; Dayel et al., 2011; Leadbeater, 2015; Laundon et al., 2019). They have been hypothesized to role as channels for intercellular communication (Fairclough et al., 2013). It has been shown that the number of intercellular bridges increases with the size of S. rosetta colonies (Laundon et al., 2019). In this study we report the presence of plasma membrane contacts between some cells of rosette colonies of Due south. rosetta. Our data show that the full number of intercellular plasma membrane contacts is comparable to the number of intercellular bridges and increases with colony size in a very similar pattern as observed for intercellular bridges (Figures 7A,B,D,E). Information technology is idea that Cadherins are central mediators of plasma membrane contacts and cell adhesion in metazoans (King et al., 2003; Cereijido et al., 2004; Halbleib and Nelson, 2006). Twenty-3 cadherins have been found in the strictly alone choanoflagellate Monosiga brevicollis. 2 of these cadherins localize in the microvillar neckband and colocalize with the actin cytoskeleton (Abedin and King, 2008). In S. rosetta, 29 proteins containing cadherin domains take been described (Nichols et al., 2012). Withal, the functions of these S. rosetta cadherins are still unknown (Fairclough et al., 2013). Some of the cadherins are differentially expressed during different stages of S. rosetta life history. Interestingly, ii of these cadherins (PTSG_06458 and PTSG_06068) are upregulated in colonies compared to single cells (Fairclough et al., 2013). Further investigation of the spatial expression patterns of these two and other cadherins are crucial to clarify the properties and potential functions of intercellular membrane contacts in colonial choanoflagellates. In dissimilarity to intercellular bridges and membrane contacts, the number of filo-/pseudopodial cell–cell contacts seems not tightly correlated with the size of a colony and might be a more than variable and transient blazon of jail cell–jail cell contacts (Figures 7C,F). It remains to be examined if some more stable types of choanoflagellate cell–prison cell contacts (intercellular bridges, plasma membrane contacts) have homologous structures in metazoans and therefore might have been nowadays in the last mutual antecedent of choanoflagellates and metazoans.
Spatial Cell Disparity and the Last Common Antecedent of Choanoflagellates and Metazoans
Our written report reports spatial cell disparity within rosette colonies of the choanoflagellate S. rosetta. The major part of this spatial prison cell disparity might be due to asynchronous cell-cycles (nuclear and jail cell volumes) and variations in metabolic processes (mitochondrial and nutrient vacuole volumes). Single choanoflagellate cells for example may only showroom cell disparity in time (life history) but not in space considering the same single prison cell can only accept one specific identity at the time (Figure 9). A choanoflagellate colony consisting of several cells can additionally exhibit cell disparity in infinite since different cells can have unlike identities. In theory, upon increase of cell numbers in a colony, increased cell identities can exist present at the aforementioned time point leading to a college possible jail cell disparity within the colony (Figure nine). However, a generalization of the thought that cell disparity increases with colony size is limited by the sample size investigated in this study. More South. rosetta colonies must exist investigated in detail to test this idea. Another aim was (three) to compare our data to previously published information on nuclear, mitochondrial, and food vacuole volumes in choanocytes of the homoscleromorph sponge O. carmela (Laundon et al., 2019). O. carmela choanocytes showroom smaller nuclear and mitochondrial just larger nutrient vacuole volumes compared to cells of S. rosetta colonies (Laundon et al., 2019, this report; Effigy viii). Additionally to these findings, nosotros showed that the maximum book difference of each of the three organelle volumes is lower compared to colonial choanoflagellate cells (Figure 8). It seems that sponge choanocytes are non just more specialized on nutrient acquisition (high volume of food vacuoles and lower mitochondrial and nuclear volumes) but also more similar to each other than individual cells in a colony of S. rosetta. Therefore, choanocytes seem to exhibit lower spatial cell disparity compared to colonial S. rosetta cells (Figure ix). Is it possible to integrate this thought into an evolutionary context to explain the origin of metazoan cell types?
In contrast to the "Blastea/Gastrea" theory (Haeckel, 1874, 1892), the Synzoospore hypothesis proposed that the origin of the Metazoa corresponds to the transition from temporal to spatial jail cell differentiation (Zakhvatkin, 1949; Mikhailov et al., 2009). Zakhvatkin (1949) suggested that the last mutual ancestor of the Metazoa might accept been an organism that already exhibited different cell types during dissimilar life history phases (temporal prison cell disparity and cell differentiation) as it tin can exist seen in many protozoan taxa such as S. rosetta (Mikhailov et al., 2009; Dayel et al., 2011). During evolution, this organism acquired a benthic colonial or multicellular phase that was made up by cells of different cell types already present in the single jail cell stages of the life history of this organism. Mikhailov et al. (2009) suggested that it is unlikely that genetic programs of jail cell differentiation evolved de novo in this terminal common ancestor of the Metazoa. Instead, pre-existing mechanisms (prison cell differentiation programs) were used to integrate the unlike cell types that already occur during unmarried prison cell life history phases of this organism. On the footing of a detailed ultra-structural study, Laundon et al. (2019) suggested that colonial cells of Due south. rosetta might stand for a distinct cell type instead of a conglomerate of identical "tiresome swimmer" cells. The carrot-shaped and chili-shaped cell may also represent singled-out cell types (Laundon et al., 2019), which is supported by our finding of loftier cell disparity in S. rosetta colonies.
Despite the controversy whether metazoans evolved from an ancestor exhibiting a "simple" or more complex life history, two chief advantages have been proposed to drive positive selection for multicellularity in general. The first is an increase of size (Bonner, 2009). Larger organisms/colonies might experience a lower predation pressure compared to smaller organisms/colonies (intercolonial competition) (Bonner, 2009). Still, later a certain size has been reached, cells within a colony might exhibit competition for space and nutrient availability (intracolonial competition). Therefore, selection might favor a amend integration of cells by colonizing unlike "niches," gradually condign more different from each other (increasing spatial jail cell disparity) and eventually are recognized as different cell types. The result might take been a multicellular organism with different cell types that exhibit division of labor (Bonner, 2009).
Recently it was shown that sponge archeocytes, and not sponge choanocytes, share like gene expression profiles with choanoflagellates (Sogabe et al., 2019), thus questioning the shut evolutionary relationship of choanoflagellates and choanocytes. In addition, sponge archaeocytes can differentiate into many other sponge prison cell types, including choanocytes and the authors suggested an alternative path to the first animals (Sogabe et al., 2019). Non a cell with a choanoflagellate morphology was the ancestral cell that preceded animal multicellularity, merely a jail cell which was able to differentiate quickly into different prison cell types, like to a stem cell institute in many different animals. Our data now hint at the possibility that likewise choanoflagellate cells have the capacity for differentiation. To further our understanding on the evolutionary origin of animate being jail cell types and cell differentiation 3D reconstructions and detailed volumetric analyses of boosted choanoflagellate cells and additional sponge choanocytes and archeocytes are needed.
Information Availability Statement
The datasets generated for this study are available on request to the corresponding writer.
Writer Contributions
BN and PB designed the study and wrote the manuscript. BN performed the experiments and analyzed the data.
Funding
This piece of work was supported by the Sars Centre core budget.
Disharmonize of Interest
The authors declare that the enquiry was conducted in the absence of any commercial or financial relationships that could be construed as a potential disharmonize of interest.
Acknowledgments
Nosotros thank the three reviewers for disquisitional reading and effective comments on this manuscript. Additionally, we thank Tarja Hoffmeyer, Ronja Goehde, and Lennart Olsson for critical reading of an earlier version of the manuscript.
Supplementary Material
The Supplementary Material for this article can exist found online at: https://www.frontiersin.org/articles/x.3389/fcell.2019.00231/full#supplementary-material
FIGURE S1 | Scheme of the workflow and software types used in this report.
Effigy S2 | 3D-surface-renderings of cells of a rosette colony of South. rosetta (RC1). Cells are not to scale. (A) 3D-view of the whole colony from different angles. The colour spectrum indicates the identity of the dissimilar cells. (B–H) Single views of cells of the colony. Cells are oriented along the apical (flagellar)–basal centrality. The volume of the whole prison cell body is given beneath every jail cell.
FIGURE S3 | 3D-surface-renderings of cells of a rosette colony of Southward. rosetta (RC2). Cells are not to scale. (A) 3D-view of the whole colony from different angles. The color spectrum indicates the identity of the different cells. (B–L) Single views of cells of the colony. Cells are oriented along the apical (flagellar)–basal axis. The book of the whole cell torso is given below every cell. C10 was not completely sectioned and one-half of the volume was added to approximate the total cellular volume indicated past the dotted line.
FIGURE S4 | 3D-surface-renderings of cells of a rosette colony of S. rosetta (RC3). Cells are not to scale. (A) 3D-view of the whole colony from different angles. The color spectrum indicates the identity of the different cells. (B–Yard) Single views of cells of the colony. Cells are oriented forth the apical (flagellar)–basal axis. The book of the whole cell body is given beneath every cell.
Figure S5 | 3D-surface-renderings of cells of a rosette colony of South. rosetta (RC4). Cells are not to scale. (A) 3D-view of the whole colony from unlike angles. The colour spectrum indicates the identity of the different cells. (B–1000) Single views of cells of the colony. Cells are oriented along the apical (flagellar)–basal centrality. The book of the whole jail cell body is given below every cell.
Figure S6 | 3D book renderings of the nucleus, mitochondrial reticulum, and food vacuoles of cells of a rosette colony of S. rosetta (RC1). (A–G) Jail cell i (C1) to cell 7 (C7). Cells are not to calibration. Cells are oriented along the upmost (flagellar)–basal axis. The prison cell body is shown half transparent. The nucleus is colored in dark greyness and the mitochondrial reticulum in brown. Food vacuoles with high electron density are colored in light greenish while food vacuoles with lower electron density are colored in nighttime grey.
Effigy S7 | 3D volume renderings of the nucleus, mitochondrial reticulum, and nutrient vacuoles of cells of a rosette colony of S. rosetta (RC2). (A–K) Cell i (C1) to jail cell 11 (C11). Cells are non to calibration. Cells are oriented along the apical (flagellar)–basal axis. The cell body is shown half transparent. The nucleus is colored in dark grayness and the mitochondrial reticulum in brown. Food vacuoles with high electron density are colored in light green while food vacuoles with lower electron density are colored in dark gray.
Effigy S8 | 3D book renderings of the nucleus, mitochondrial reticulum, and food vacuoles of cells of a rosette colony of Southward. rosetta (RC3). (A–50) Jail cell one (C1) to prison cell 12 (C12). Cells are not to scale. Cells are oriented along the apical (flagellar)–basal axis. The prison cell body is shown one-half transparent. The nucleus is colored in dark gray and the mitochondrial reticulum in brownish. Food vacuoles with loftier electron density are colored in light green while nutrient vacuoles with lower electron density are colored in dark greyness.
Figure S9 | 3D volume renderings of the nucleus, mitochondrial reticulum, and nutrient vacuoles of cells of a rosette colony of S. rosetta (RC4). (A–J) Cell one (C1) to cell ten (C10). Cells are not to scale. Cells are oriented forth the apical (flagellar)–basal axis. The cell trunk is shown half transparent. The nucleus is colored in nighttime gray and the mitochondrial reticulum in brownish. Food vacuoles with high electron density are colored in light light-green while food vacuoles with lower electron density are colored in night grayness.
Footnotes
- ^ 10.6084/m9.figshare.7346750.v2
References
Adl, South. K., Bass, D., Lane, C. Due east., Lukeš, J., Schoch, C. 50., Smirnov, A., et al. (2019). Revisions to the nomenclature, nomenclature, and variety of eukaryotes. J. Eukaryot. Microbiol. 66, 4–119. doi: 10.1111/jeu.12691
PubMed Abstract | CrossRef Full Text | Google Scholar
Alberts, B., Johnson, A., Lewis, J., Morgan, D., Raff, M., Roberts, K., et al. (2014). Molecular Biology of the Prison cell, sixth Edn. New York, NY: Norton & Visitor.
Google Scholar
Alegado, R. A., Brown, 50. W., Cao, Due south., Dermenjian, R. Thousand., Zuzow, R., Fairclough, Due south. R., et al. (2012). A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals. eLife 1:e00013. doi: 10.7554/eLife.00013
PubMed Abstract | CrossRef Full Text | Google Scholar
Arendt, D., Musser, J. Thousand., Bakery, C. V., Bergman, A., Cepko, C., Erwin, D. H., et al. (2016). The origin and evolution of jail cell types. Nat. Rev. Genet. 17, 744–757. doi: ten.1038/nrg.2016.127
PubMed Abstract | CrossRef Total Text | Google Scholar
Bonner, J. T. (2009). Start Signals: The Evolution of Multicellular Development. Princeton, NJ: Princeton University Press.
Google Scholar
Booth, D. S., Szmidt-Middleton, H., and King, N. (2018). Transfection of choanoflagellates illuminates their cell biology and the ancestry of animal septins. Mol. Biol. Cell 29, 3026–3038. doi: x.1091/mbc.E18-08-0514
PubMed Abstract | CrossRef Full Text | Google Scholar
Carr, M., Leadbeater, B. Due south., Hassan, R., Nelson, Thousand., and Baldauf, Due south. L. (2008). Molecular phylogeny of choanoflagellates, the sister group to Metazoa. Proc. Natl. Acad. Sci. U.S.A. 105, 16641–16646. doi: 10.1073/pnas.0801667105
PubMed Abstract | CrossRef Full Text | Google Scholar
Dayel, M. J., Alegado, R. A., Fairclough, S. R., Levin, T. C., Nichols, S. A., McDonald, K., et al. (2011). Cell differentiation and morphogenesis in the colony-forming choanoflagellate Salpingoeca rosetta. Dev. Biol. 357, 73–82. doi: x.1016/j.ydbio.2011.06.003
PubMed Abstract | CrossRef Full Text | Google Scholar
Ereskovsky, A. V., Richter, D. J., Lavrov, D. V., Schippers, Grand. J., and Nichols, Due south. A. (2017). Transcriptome sequencing and delimitation of sympatric Oscarella species (O. carmela and O. pearsei sp. nov) from California, USA. PLoS One 12:e0183002. doi: x.1371/periodical.pone.0183002
PubMed Abstract | CrossRef Total Text | Google Scholar
Fairclough, S. R., Chen, Z., Kramer, Due east., Zeng, Q., Young, S., Robertson, H. Chiliad., et al. (2013). Premetazoan genome evolution and the regulation of prison cell differentiation in the choanoflagellate Salpingoeca rosetta. Genome Biol. fourteen:R15. doi: ten.1186/gb-2013-fourteen-2-r15
PubMed Abstruse | CrossRef Full Text | Google Scholar
Fairclough, Southward. R., Dayel, M. J., and Rex, N. (2010). Multicellular development in a choanoflagellate. Curr. Biol. twenty, R875–R876.
Google Scholar
Gilbert, S. F. (2013). Developmental Biology. Basingstoke: Palgrave.
Google Scholar
Haeckel, Eastward. (1874). Die Gastrea-Theorie, die phylogenetische Klassifikation des Tierreichs, und die Homologie der Keimblätter. Jenaische Zeitschrift für Naturwissenschaften 8, ane–55.
Google Scholar
Haeckel, E. (1892). The History of Creation: or The Development of the World and its Inhabitants past the Action of Natural Causes, Vol. 2. New York, NY: D. Appleton & Co.
Google Scholar
Jevtić, P., Edens, L. J., Vuković, Fifty. D., and Levy, D. L. (2014). Sizing and shaping the nucleus: mechanisms and significance. Curr. Opin. Cell Biol. 28, xvi–27. doi: 10.1016/j.ceb.2014.01.003
PubMed Abstract | CrossRef Total Text | Google Scholar
Karpov, S. A., and Coupe, South. J. (1998). A revision of choanoflagellate genera Kentrosiga Schiller, 1953 and Desmarella Kent, 1880. Acta Protozool. 37, 23–28.
Google Scholar
King, N., Hittinger, C. T., and Carroll, S. B. (2003). Development of key prison cell signaling and adhesion poly peptide families predates beast origins. Science 301, 361–363. doi: 10.1126/science.1083853
PubMed Abstract | CrossRef Total Text | Google Scholar
Laundon, D., Larson, B., McDonald, K., Rex, N., and Burkhardt, P. (2019). The architecture of cell differentiation in choanoflagellates and sponge choanocytes. PLoS Biol. 17:e3000226. doi: ten.1371/journal.pbio.3000226
PubMed Abstruse | CrossRef Total Text | Google Scholar
Leadbeater, B. S. C. (2015). The Choanoflagellates. Cambridge: Cambridge University Press.
Google Scholar
Levin, T. C., Greaney, A. J., Wetzel, L., and King, N. (2014). The Rosetteless gene controls development in the choanoflagellate S. rosetta. eLife 3:e04070. doi: x.7554/eLife.04070
PubMed Abstract | CrossRef Full Text | Google Scholar
Maeshima, G., Iino, H., Hihara, S., and Imamoto, N. (2011). Nuclear size, nuclear pore number and jail cell cycle. Nucleus 2, 1065–1071.
Google Scholar
Mah, J. L., Christensen-Dalsgaard, Thousand. K., and Leys, South. P. (2014). Choanoflagellate and choanocyte neckband-flagellar systems and the supposition of homology. Evol. Dev. 16, 25–37. doi: ten.1111/ede.12060
PubMed Abstract | CrossRef Full Text | Google Scholar
Mikhailov, K. V., Konstantinova, A. V., Nikitin, Thou. A., Troshin, P. V., Rusin, Fifty. Y., Lyubetsky, Five. A., et al. (2009). The origin of Metazoa: a transition from temporal to spatial cell differentiation. Bioessays 31, 758–768. doi: 10.1002/bies.200800214
PubMed Abstract | CrossRef Full Text | Google Scholar
Nichols, Due south. A., Roberts, B. West., Richter, D. J., Fairclough, S. R., and King, North. (2012). Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/β-catenin complex. Proc. Natl. Acad. Sci. U.South.A. 109, 13046–13051. doi: x.1073/pnas.1120685109
PubMed Abstract | CrossRef Full Text | Google Scholar
Rozwadowska, Northward., Kolanowski, T., Wiland, Due east., Siatkowski, One thousand., Pawlak, P., Malcher, A., et al. (2013). Characterisation of nuclear architectural alterations during in vitro differentiation of human stem cells of myogenic origin. PLoS One 8:e73231. doi: 10.1371/periodical.pone.0073231
PubMed Abstract | CrossRef Full Text | Google Scholar
Ruiz-Trillo, I., Roger, A. J., Burger, G., Gray, One thousand. West., and Lang, B. F. (2008). A phylogenomic investigation into the origin of metazoa. Mol. Biol. Evol. 25, 664–672. doi: 10.1093/molbev/msn006
PubMed Abstract | CrossRef Full Text | Google Scholar
Sebé-Pedrós, A., Chomsky, East., Pang, Thousand., Lara-Astiaso, D., Gaiti, F., Mukamel, Z., et al. (2018a). Early metazoan prison cell type diverseness and the evolution of multicellular cistron regulation. Nat. Ecol. Evol. 2, 1176–1188. doi: 10.1038/s41559-018-0575-half dozen
PubMed Abstract | CrossRef Full Text | Google Scholar
Sebé-Pedrós, A., Saudemont, B., Chomsky, E., Plessier, F., Mailhé, K.-P., Renno, J., et al. (2018b). Cnidarian prison cell type diversity and regulation revealed by whole-organism single-jail cell RNA-Seq. Cell 173, 1520.–1534. doi: x.1016/j.cell.2018.05.019
PubMed Abstruse | CrossRef Full Text | Google Scholar
Shore, R. E. (1971). Growth and renewal studies of the choanocyte population in Hymeniacidon sinapium (Porifera: Demospongiae) using colcemid and iii-H thymidine. J. Exp. Zool. 177, 359–363. doi: 10.1002/jez.1401770310
CrossRef Total Text | Google Scholar
Simpson, T. L. (2012). The Cell Biology of Sponges. Berlin: Springer Scientific discipline & Business Media.
Google Scholar
Sogabe, S., Hatleberg, W. 50., Kocot, G. 1000., Say, T. E., Stoupin, D., Roper, K. E., et al. (2019). Pluripotency and the origin of animal multicellularity. Nature 570, 519–522. doi: x.1038/s41586-019-1290-four
PubMed Abstract | CrossRef Total Text | Google Scholar
Wagner, G. P. (2014). Homology, Genes, and Evolutionary Innovation. Princeton, NJ: Princeton University Press.
Google Scholar
Wetzel, Fifty., Levin, T. C., Hulett, R. E., Chan, D., Male monarch, Thou. A., Aldayafleh, R., et al. (2018). Predicted glycosyltransferases promote evolution and forestall spurious cell clumping in the choanoflagellate S. rosetta. eLife 7:e41482. doi: 10.7554/eLife.41482
PubMed Abstract | CrossRef Total Text | Google Scholar
Woznica, A., Cantley, A. M., Beemelmanns, C., Freinkman, E., Clardy, J., and Male monarch, N. (2016). Bacterial lipids activate, synergize, and inhibit a developmental switch in choanoflagellates. Proc. Natl. Acad. Sci. UsaA. 113, 7894–7899. doi: 10.1073/pnas.1605015113
PubMed Abstruse | CrossRef Full Text | Google Scholar
Zakhvatkin, A. A. (1949). The Comparative Embryology of the Depression Invertebrates. SOURCES and Method of the Origin of Metazoan Development. Moscow: Soviet Science, 395.
Google Scholar
Zhou, J., Damdimopoulos, A. E., Spyrou, 1000., and Brüne, B. (2007). Thioredoxin 1 and thioredoxin 2 have opposed regulatory functions on hypoxia-inducible factor-1α. J. Biol. Chem. 282, 7482–7490. doi: ten.1074/jbc.m608289200
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fcell.2019.00231/full
0 Response to "what would happen to a cell of a colonial organism if you separated it from the other cells?"
ارسال یک نظر